CHPS RESEARCH STUDIES
The Influence of Oral Contraceptives During Disuse
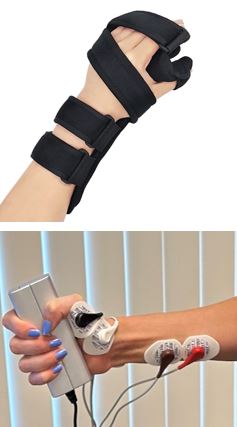
Studies across various sports and physical activities have consistently shown that females are at greater risk than their male counterparts for musculoskeletal injuries. We are doing this study to see if there may be a link between sex hormones and injury risk and if the immobilization of your wrist joint affects your muscle strength. We will compare your results with other participants to see if the changes in muscle strength are linked to differences in gender and sex related hormones. We will compare results from men, women on oral contraceptives, and women who are not taking oral contraceptives.
Inclusion Qualifications:
- Right-handed
- Age between 18-35 years
- Males
- Females that have consistently used monophasic oral contraceptives for the previous 6 months
- Females that have not used any form of contraceptives for the previous 6 months
Exclusion Qualifications:
You may not participate in this research opportunity if any of the following applies to you:- Gender identity inconsistent with biological sex (due to the influence that drugs used for gender reassignment may have)
- Females that are amenorrheic (lack of menstruation for ≥3 consecutive cycles) or oligomenorrheic (menstrual cycle length ≤36 days)
- Menstrual cycle irregularities among females not using OCs (regularity defined as every 21 to 35 days and/or at least 5 periods in the last six months)
- Any contraceptive use other than monophasic OC within the last 6 months
- Monophasic OC that has been inconsistent over the previous 6 months
- Score of >2/5 on question 9, or a score of >1/5 on question 10 or 11 on the quick DASH outcome measure indicating pain/discomfort of the upper extremities (shoulder, elbow, wrist, hand)
- Dominant hand is the left hand
- Body Mass Index < 18.5 kg/m2 or > 29.9 kg/m2
- Current depression or anxiety
- History of musculoskeletal injury, pain, or surgery of the elbow, wrist, or hand
- Unwillingness to avoid upper-body exercise during Phase 1
- Unwillingness to avoid exercise and alcohol 24 hours prior to each testing visit.
- Neuromuscular disease (e.g., MS, ALS, Parkinson’s)
- Metabolic disease (e.g., diabetes, thyroid disorder, metabolic syndrome)
- Personal or family history of blood clots
- Trouble using or controlling one’s muscles
- History of cancer
- History of stroke
- History of heart attack
- History of arthritis
- Allergy to rubbing alcohol
- Lack of transportation to and from the laboratory
- Current or planned pregnancy (within the next three months)
- Implant of any kind
- The use of medications that may increase the risk of blood clots (i.e., corticosteroids such as prednisone, testosterone and anabolic steroids, selective estrogen receptor modulators like tamoxifen, aromatase inhibitors including anastrozole, thalidomide and lenalidomide, erythropoiesis-stimulating agents such as epoetin alfa, antipsychotics like chlorpromazine, antidepressants including paroxetine, various cancer chemotherapies, immune modulating drugs, antivirals such as ritonavir, and tamoxifen).
- The use of creatine monohydrate and beta alanine supplementation within the previous 6 months.
- The use of any medication or supplement that could influence hormone levels within the previous 6 months.
Get Notified of Future Opportunities
Subscribe to our email list to be automatically notified of all future research participation opportunities as soon as they become available.
Recruitment End Date:
July 17, 2024
July 17, 2024
Location:
Health Sciences 1
12805 Pegasus Drive
258
Orlando, FL 32816
Health Sciences 1
12805 Pegasus Drive
258
Orlando, FL 32816
Time Commitment:
Number of visits: 3
Expected time per visit: 1 hours
Number of visits: 3
Expected time per visit: 1 hours
Compensation:
Type: Up to $100 from Amazon
Type: Up to $100 from Amazon
Point of Contact:
Kelsey Stambaugh
Kelsey Stambaugh
Associated Units:
Kinesiology
Physical Therapy
Exercise Physiology & Rehabilitation Science
Kinesiology
Physical Therapy
Exercise Physiology & Rehabilitation Science